Now Enrolling: A clinical trial with the potential to improve outcomes for patients with triple-negative breast cancer who have received prior therapies
Currently Enrolling Participants with Recurrent Triple-Negative Breast Cancer
MER-XMT-1660-1: A Clinical Trial of Emiltatug Ledadotin (Emi-Le; XMT-1660); Dose Expansion Cohorts
Trial Information
An antibody-drug-conjugate, or ADC for short, is a type of drug designed to target and kill cancer cells expressing a specific antigen, or biological marker (otherwise known as a biomarker).
ADCs are different from traditional chemotherapy drugs.
Emiltatug ledadotin (also known as Emi-Le or XMT-1660) is an investigational ADC that is designed to target cancer cells expressing the biomarker B7-H4. Emi-Le is currently not approved by the FDA or any other regulatory authority for the treatment of cancer.
The ongoing clinical trial is evaluating the safety and efficacy of Emi-Le in adult patients with recurrent advanced or metastatic triple-negative breast cancer.
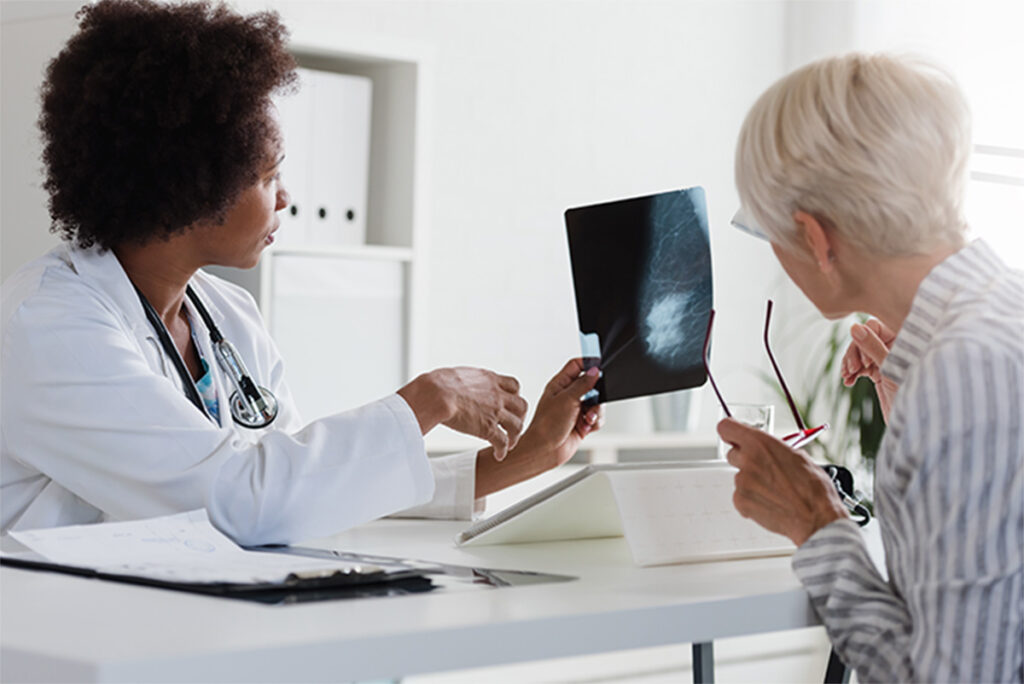
Now enrolling Part 2: This is the dose expansion portion of a Phase 1 trial evaluating the safety and efficacy of Emi-Le in patients with recurrent advanced or metastatic triple-negative breast cancer who have received 1-4 prior therapies, including at least one prior ADC.
For more information about the trial, a list of hospitals or institutions participating in this clinical trial and/or information about your eligibility, please visit clinicaltrials.gov, discuss the trial with your doctor and/or click here to send an email to Emi-Le@mersana.com.
